
上海胤煌科技有限公司-傘棚燈、不溶性微粒
 金牌會員
金牌會員 已認證
已認證


上海胤煌科技有限公司-傘棚燈、不溶性微粒
 金牌會員
金牌會員 已認證
已認證
What is Zeta Potential / 什么是Zeta電位?
1. 什么是Zeta電位?
Zeta potential is an electrostatic potential that exists very near the surface of particles suspended in liquids1. Zeta potential (ζ) is responsible for particle-particle repulsion forces in colloidal suspensions and thus can be used to predict colloid stability against particle aggregation. Figure 1 illustrates a particle suspended in a liquid along with various notional regions around it. The“slipping plane” or “shear plane” is where Zeta potential is located versus the potential in the bulk solution. Within this slipping plane, the liquid is bound to the particle while it moves freely outside this boundary. The net potential far from the particle (in the bulk of the liquid) is zero.
Zeta電位是液體中懸浮的粒子很接近表面位置的靜電勢1。Zeta電位(ζ)是由膠體中粒子與粒子間的相互作用造成的,因此它可以用來預測膠體體系里粒子聚集的穩定性。圖1顯示了懸浮在液體中的粒子及其周圍的各種概念區域。Zeta電位指的是液體中滑動面或者剪切面的電位。在這個滑動平面內,當液體在這個邊界外自由運動時,它與粒子結合在一起。遠離粒子的凈電勢(在液體中)為零。
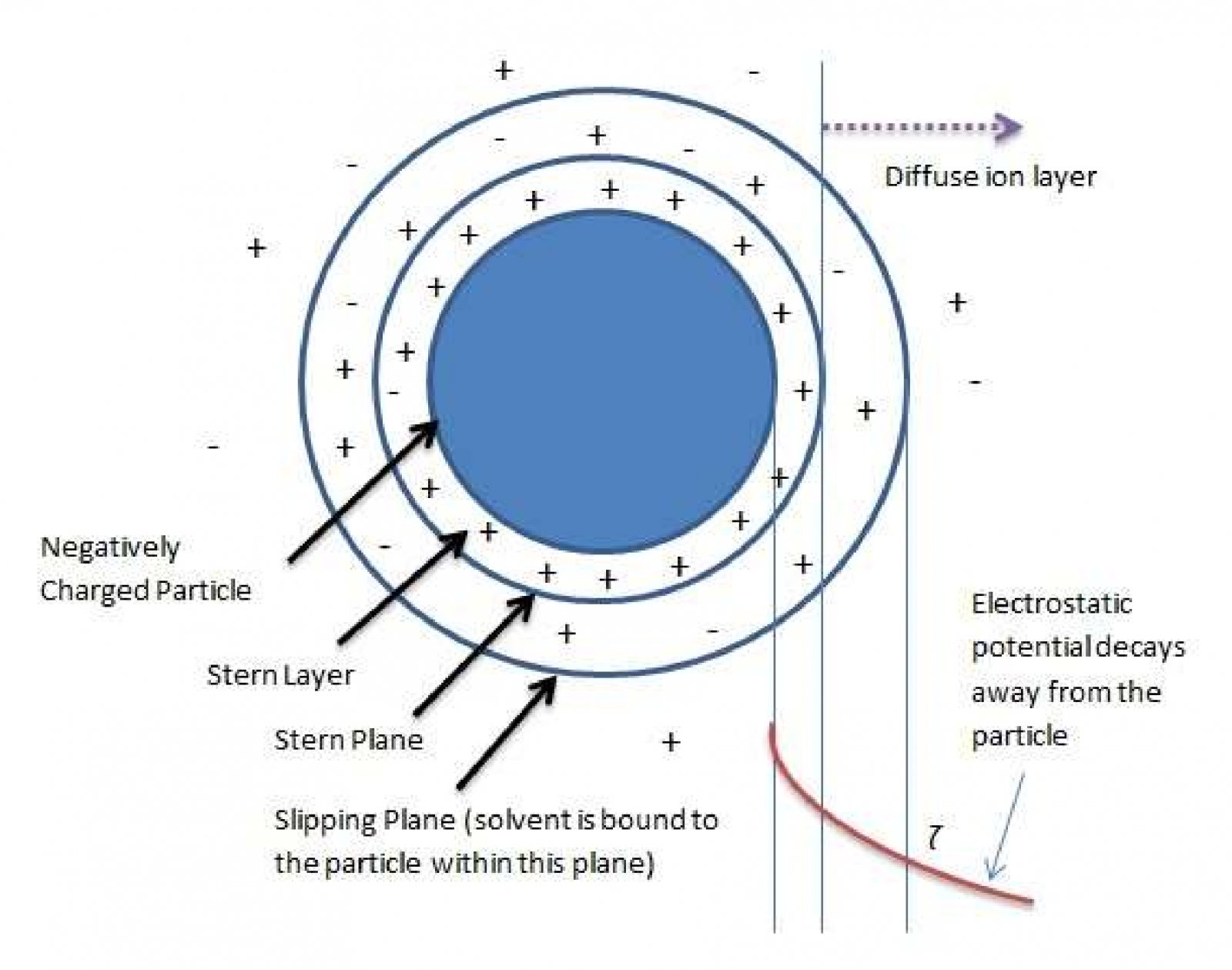
Figure 1. A negatively charged particle suspended in a liquid. Notional boundaries are shown.
圖1懸浮在液體中的帶負電的粒子及其周圍的各種概念區域
PS:這個是另外的一種說法,但是要描述的內容是一樣的;
由于分散粒子表面帶有電荷而吸引周圍的反號離子,這些反號離子在兩相界面呈擴散狀態分布而形成擴散雙電層。根據Stern雙電層理論可將雙電層分為兩部分,即Stern層和擴散層。Stern層定義為吸附在電極表面的一層離子(IHP or OHP)電荷中心組成的一個平面層,此平面層相對遠離界面的流體中的某點的電位稱為Stern電位。穩定層(Stationary layer) (包括Stern層和滑動面slipping plane以內的部分擴散層) 與擴散層內分散介質(dispersion medium)發生相對移動時的界面是滑動面(slipping plane),該處對遠離界面的流體中的某點的電位稱為Zeta電位或電動電位(ζ-電位)。
2. 測Zeta電位為什么不能稀釋?
In aqueous media, Zeta potential is typically generated as the ions on the particle surface dissociate, leaving a net electric charge near the surface surrounded by a cloud of counter-ions. Various types of ions can diffuse in and out through the slipping plane which allows Zeta potential to vary depending on the ion composition in the liquid such as pH. Ions may also participate in chemical reactions within the slipping plane which can affect the Zeta potential. Sample dilution can significantly shift the Zeta potential as ions may adsorb or desorb from the particle. Thus, Zeta potential can be positive or negative, or zero (Iso-Electric Point, IEP) depending on the liquid (solvent) pH or ion type and concentration.
在水相介質中,Zeta電位通常是由于粒子表面的離子離解而產生的,在表面附近留下一個被反離子云包圍的凈電荷。各種類型的離子可以通過滑動面擴散進來和出去,滑動面允許Zeta電位根據液體中的離子組成而變化,例如pH值。離子也可以通過參與滑動面內的化學反應,從而影響Zeta電位。樣品稀釋可以顯著地改變Zeta電位,因為離子可以吸附或者解析顆粒。因此,Zeta電位可以是正的或負的,也可以是零(等電點,IEP),這取決于液體(溶劑)的pH值或離子的類型和濃度。
3. 測量Zeta電位的方法
Particle-filtration systems may benefit from low Zeta potential levels as aggregated particles are easier to remove. Most other colloidal systems require higher Zeta potentials, e.g. over +/- 20 millivolts in order to maximize shell life. Coatings tend to be more efficient when the particles and coated surface have opposite polarities. Zeta potential normally cannot be directly measured. For example, one cannot place a voltmeter probe against a particle surface in order to measure its surface potential. Instead, Zeta potential is calculated from electrophoretic measurements which measure particle velocity under an applied electric field, i.e. make the particles move and measure their particle mobility (see ). Thus, the calculated Zeta potential depends on the theory used in these computations to relate particle mobility to Zeta potential. An alternative measurement for large particles or surfaces is to move the liquid against stationary particles, fibers, or surfaces and measure the resulting streaming potential。
顆粒過濾系統可能受益于較低的Zeta電位水平,因為聚集顆粒更容易去除。大多數其他膠體系統需要較高的Zeta電位,例如超過+/- 20毫伏,以最大限度地提高殼體壽命。當顆粒和涂層表面具有相反的極性時,涂層往往更有效。Zeta電位通常不能直接測量。例如,不能將伏特計探頭靠在粒子表面上以測量其表面電位。相反,Zeta電位是通過電泳測量來計算的,電泳測量是在外加電場下測量粒子速度,也就是通過粒子移動并測量其粒子遷移率(見)。因此,計算出的Zeta電位取決于這些計算中使用的理論,即粒子遷移率與Zeta電位的關系。另一種測量大顆粒或表面電位的方法是將液體移到靜止的顆粒、纖維或表面上,然后測量產生的流動電位。
4. 電位滴定法測量Zeta電位和PH的關系
Potentiometric Titrations are useful for determining a sample's IEP. As mentioned above, the IEP may be desirable or otherwise. Potentiometric Titration plots may display plateau regions for Zeta potential vs. pH. Such measurements enable manufacturers to optimize use of acids or bases for transportation and storage. Figure 2 shows a potentiometric titration on a Ludox-TM silica sample by automatic addition of 1N HCl. This titration was performed automatically by a Zeta-APS, Zeta Acoustic Particle Sizer, instrument from Matec Applied Sciences, Northborough, MA USA 3. Figure 2 shows that below pH 4, the Zeta curve approaches a plateau region while Conductivity increases more rapidly. This suggests that the silica particles are becoming saturated with H+ ions as the pH is lowered. Conductivity increases more rapidly as more of these H+ ions stay in the continuous water solvent as opposed to diffusing through the slipping plane toward the particle surface.
電位滴定法可用于測定樣品的等電點。如上所述,IEP可能是可取的或者相反。電位滴定圖可以顯示Zeta電位與pH值之間的關系變化。這樣的測量使制造商通過酸或堿的使用,優化產品的運輸和儲存。圖2顯示在Ludox TM二氧化硅樣品上自動添加1N HCl時,Zeta電位及電導率的變化。該過程是由美國MAS公司生產的超聲電聲法粒度及Zeta電位分析儀Zeta-APS設備完成的。圖2顯示,在pH<4時,Zeta曲線接近一個穩定區域,而電導率增加得更快。這表明,隨著pH值的降低,二氧化硅顆粒逐漸被H+離子飽和。當更多的H+離子停留在連續的水溶劑中時,電導率增加得更快,而不是通過滑面向顆粒表面擴散。
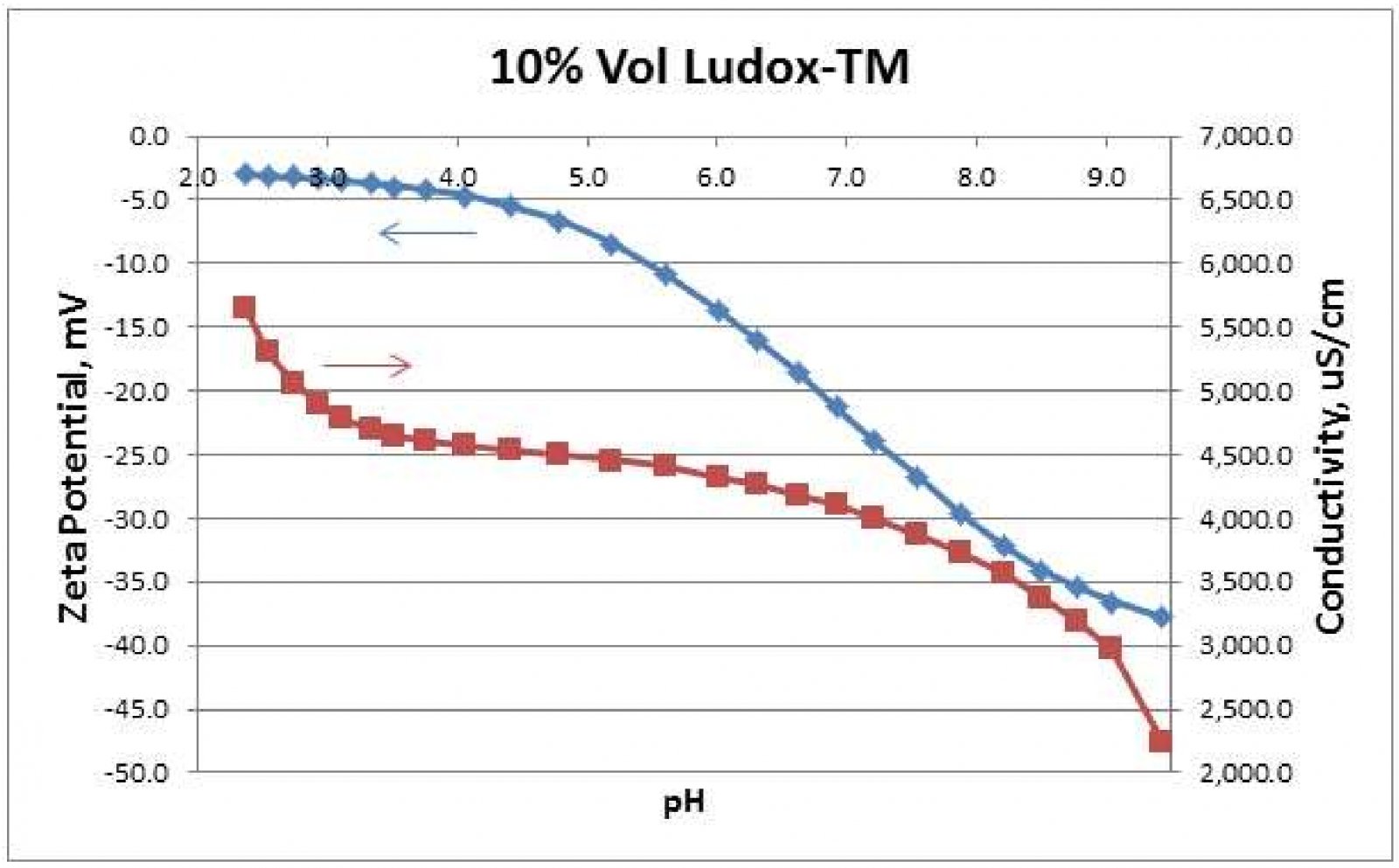
Figure 2. Automatic potentiometric titration of 10%-vol Ludox-TM by 1N HCl addition.
圖2通過自動電位滴定法將1N HCl加入到10%體積的Ludox TM二氧化硅溶液中
5. 體積滴定法測定Zeta電位和添加劑的關系
The Zeta-APS instrument is also capable of performing automatic Volumetric titrations whereby a reagent such as a surfactant is added into a colloid in dosages as small as 1 μL. The Zeta-APS then produces titration graphs showing plots such as Zeta potential, pH, Conductivity, and Temperature vs. added reagent volume. Plots of Zeta vs. reagent volume would be flat if the added surfactant is not adsorbed by the particles, i.e. it does not supply potential-determining ions1-2.
Zeta-APS儀器還能夠執行自動體積滴定,即將表面活性劑等試劑以1μL的劑量添加到膠體中。Zeta-APS隨后生成滴定圖,顯示Zeta電位、pH值、電導率和溫度與添加的試劑體積的關系圖。如果添加的表面活性劑未被顆粒吸附,則它沒有提供改變電位的粒子1-2,則Zeta與試劑體積的關系圖將是穩定的。
References
1. Hunter, R. J., Introduction to Modern Colloid Science, Oxford Science Pub., 1993.
2. Morrison, I. D., Sydney, R., Colloidal Dispersions. Suspensions, Emulsions, and Foams. John Wiley and Sons, 2002.
相關產品
更多
相關文章
更多
技術文章
2025-05-27技術文章
2025-05-20技術文章
2025-05-14技術文章
2025-05-12
虛擬號將在 秒后失效
使用微信掃碼撥號